PEMION+™ - PP1-HNN8-00
- High conductivity binder and coating material
- High concentration of functional groups
- Chemical and oxidative stability
Product Description
PEMION+™ - PP1-HNN8-00 Ionomer can be used as a coating as well as a binder. Ink formulations can be prepared to be used in fuel cell catalyst layers. Commonly the Ionomer is being mixed with a suitable alcohol that is then stirred and poured dropwise with an appropriate mass of catalyst powder and water.
Typical applications can be found in Metal Air, Nickel metal Hydride and Solid state battery chemistries. This highly conductive binder material has an affinity for negatively charged electrode constituents. It is processable in low boiling solvents for use as alkaline/acid stable electrode coating or binder and is highly customizable for optimized electrochemical properties, application size and manufacturing methods.
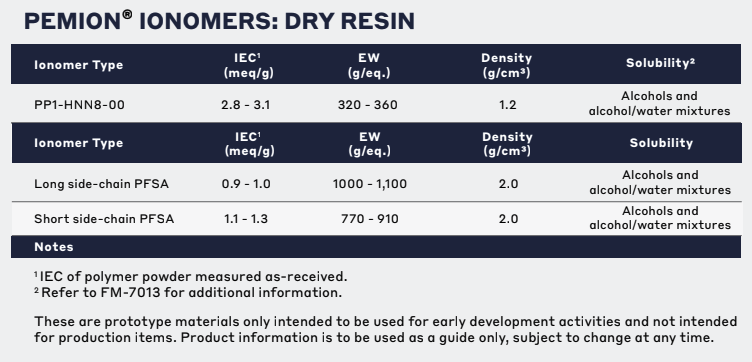
Technical Specifications
| General Properties | |
| Density (g) | 1.2 g/cm3 |
| Chemical Properties | |
| Ion Exchange Capacity (IEC) Ion Exchange Capacity (IEC) Ion-exchange capacity measures the ability of a material to undergo displacement of ions, previously attached into its structure, by oppositely charged ions. It is measured as the quantity of ions that can pass through a specific volume and a common unit is eq/L. In the case of an Ion-exchange polymer, it represents the total of active sites or functional groups responsible for the exchange and is the theoretical maximum amount of ions that we can load. | 2.8 - 3.1 meq/g |
| Other Properties | |
| Storage Temperature | Ambient °C |
Additional Information
Role of Ion Exchange Polymers in Membrane Electrode Assemblies of Water Electrolyzer and Fuel Cells
Membrane electrode assembly (MEA) is considered the heart of water electrolyzers and fuel cells. It is composed of the gas diffusion layers (or porous transport layers), catalyst layers (CLs), and ion exchange membranes. MEA contains the sites where the significant electrochemical reactions occur. They are also responsible for the mass transport processes that involve transfer of reactants into and products out of the device, as well as electron conduction from the electrodes to the external circuitry of the water electrolyzers and fuel cells. Water electrolyzer and fuel cell performance is considerably affected by the properties of GDLs, CLs, and membranes comprising the MEA. The electrodes should have high electrical conductivity and porosity, whereas the CL should exhibit high catalytic activity. Ideally, MEAs have all of these properties while being inexpensive. For polymer electrolyte membrane water electrolyzers and fuel cells specifically, cost has been an issue since the highly acidic operating conditions necessitates the usage of expensive platinum group metal catalysts, commonly Pt/C.
Balancing out cost effectiveness and device performance requires optimization of the MEA fabrication. MEAs are typically produced through two major techniques. First, the gas diffusion electrode (GDE) method involves depositing the catalyst layer on the GDL. Second, in the catalyst-coated membrane (CCM) technique, the catalyst is directly coated onto the ion exchange membranes through electrostatic spray coating, ultrasonic coating, decal transfer, and screen printing. The CCM method yields MEAs with lower interfacial resistance owing to reduced reactant diffusion path and enlarged contact area. The caveat is that the direct deposition of the catalyst on the membranes may form cracks in the CL owing to the high tendency of the catalyst particles to agglomerate and the evaporation of the solvent used in the ink formulation.
To improve catalyst utilization, ion exchange polymers or ionomers are added into catalyst ink formulations. Catalyst ink formulations are typically composed of the catalyst, a solvent, and the ion exchange polymer or ionomer. The electrochemical properties, coating characteristics, and coating stability of the CL is highly dependent on the interactions of these three primary components. Ion exchange polymers are adsorbed on the catalyst particles in the CL. This adsorption increases the surface charge of the catalyst particle agglomerates. As the electrostatic repulsion between the catalyst particles dominates over the van der Waals forces, the tendency of forming agglomerates is reduced. On top of that, the ion exchange polymer particles increase the steric hindrance, which keeps CL particles away from each other. Aside from improving catalyst utilization, the ion exchange polymer also improves charge (anion or proton) conduction as it acts as an extended charge conductor from the bulk of the ion exchange membrane. The ionomer also serves as a binder for the catalyst particles, ensuring that there are available reactant transfer and electrical conduction pathways. Lastly, the ion exchange polymers act as an hydrophilic agent, retaining the optimum moisture level to ensure that the membrane is well-hydrated.
Solvent compatibility with Ion exchange polymers
The way the ionomer interacts with the solvent and the catalyst is a key factor that determines the stability of dispersion and the rheological properties of the ink. Dissolution of Pemion® ionomer is typically achieved following stirring and gentle heating (e.g., 300–600 rpm at 60 °C) in a chosen solvent for 24–72 h, depending on vessel size, concentration, and amount of ionomer being dissolved. Filtration of the polymer solution following dissolution is recommended.
| Solvent Type | Comments | Solubility wt% |
| Methanol | Low-boiling solvent for spray coating, electrode | 1% – 10% |
| Reagent Alcohol(85% EtOHl/ 5% MeOH/ 5% isopropanol) | Low-boiling solvent for spray coating, electrode | 1% - 10% |
| Ethanol | Low-boiling solvent for spray coating, electrode | 1% - 7% |
| Ethanol/IPA (50:50 by weight) | High boiling point solvents, can cause complications with | 1% - 5% |
| IPA/water (50:50 by volume) | Low-boiling solvent for spray coating, electrode | 1% – 10% |
| DMSO | Recommended high boiling point solvent to achieve high | 1% - 10% |
We sell our ionomers in pure polymer powder form and provide comprehensive handling and dissolution instructions for customers to create their own solutions from. This saves both us and you the headache in terms of shipping flammable liquids, and the associated safety and cost considerations.
Both the PEM and AEM are readily soluble in low boiling point, common laboratory solvents.
These are prototype materials only intended to be used for early development activities and not intended for production items. Product information is to be used as a guide only, subject to change at any time.



