Fuel cells. No matter how familiar you are with them, reading their name always brings images from a distant future. They’ve been used in numerous sci-fi movies, books and video games to portray clean and limitless energy that powers up the cities of the future and our martian colonies. They’ve also been a highly controversial news topic since they have been the technology of the future for the last 30 years. As controversial as this sounds, it is absolutely true. They’ve been around since 1839 with various iterations coming in and out of fashion.
Fuel cells are being used in pilot projects and can power up high performance cars and applications. But why are they still “under development”? What keeps them from being the technology of the future and what can we do to bring the future closer to us? That’s what we are going to discuss in this quick ELI5 of fuel cells. What are they? How do they work? What kinds of products and technologies are coming into play to create energy out of thin air?
Like batteries, fuel cells are energy converters – they use an electrochemical reaction to take the chemical energy stored in a fuel source and convert it to electricity. Unlike batteries, which contain a fixed supply of energy, fuel cells do not require recharging. As long as fuel is continuously supplied to the fuel cell, electricity, water and heat will be produced
How does a fuel cell work?
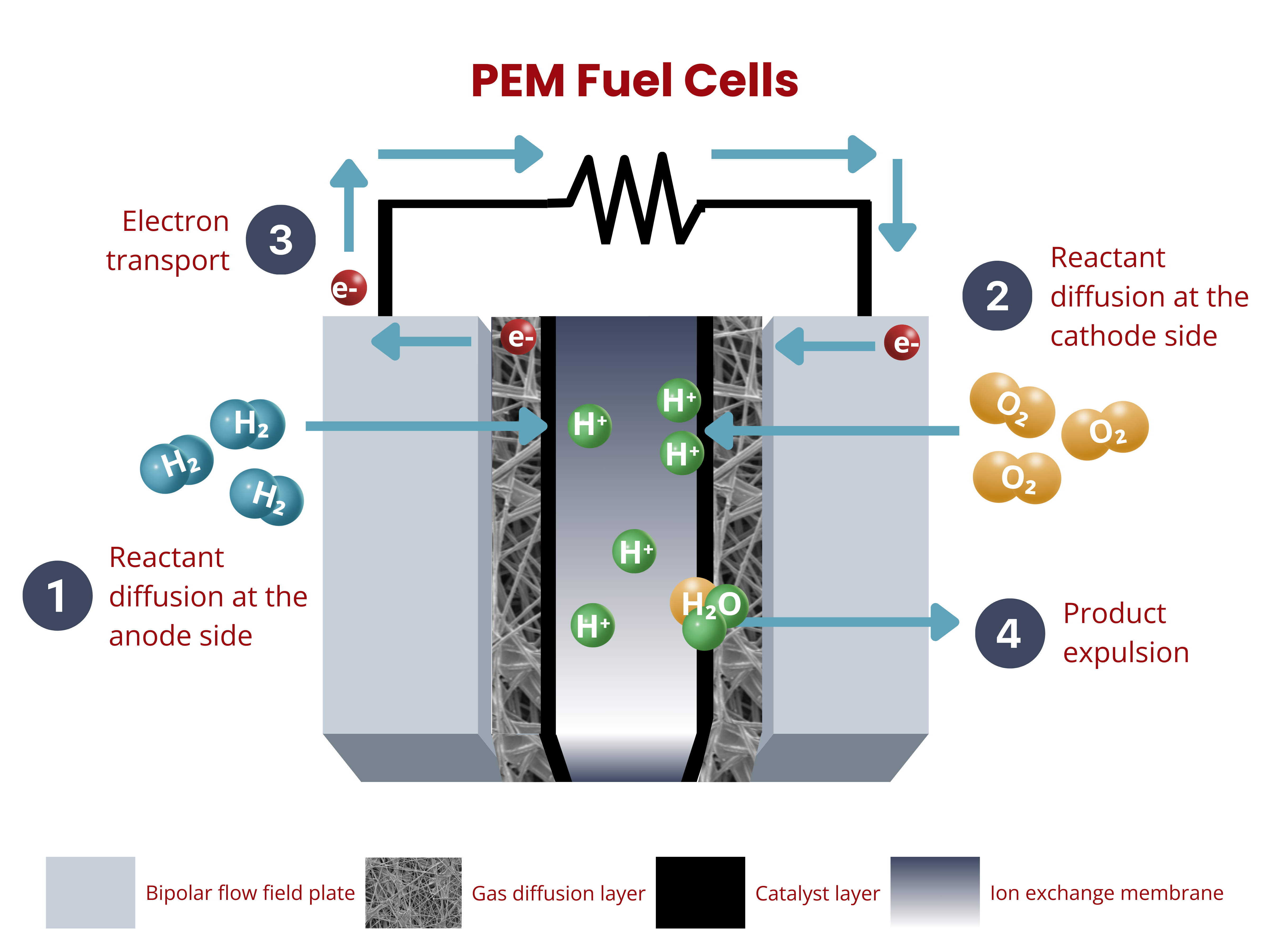
So, how does a fuel cell work? In principle the concept of a fuel cell is pretty simple.
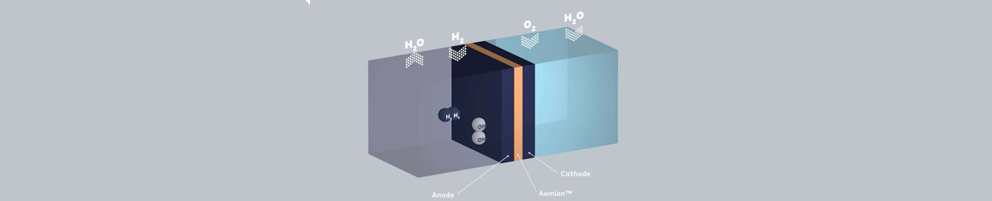
- Hydrogen gets in from anode side
- Oxygen comes in from the cathode side
- A couple of reduction and oxidation reactions happen within the system.
- Electricity and other byproducts such as heat and water come out.
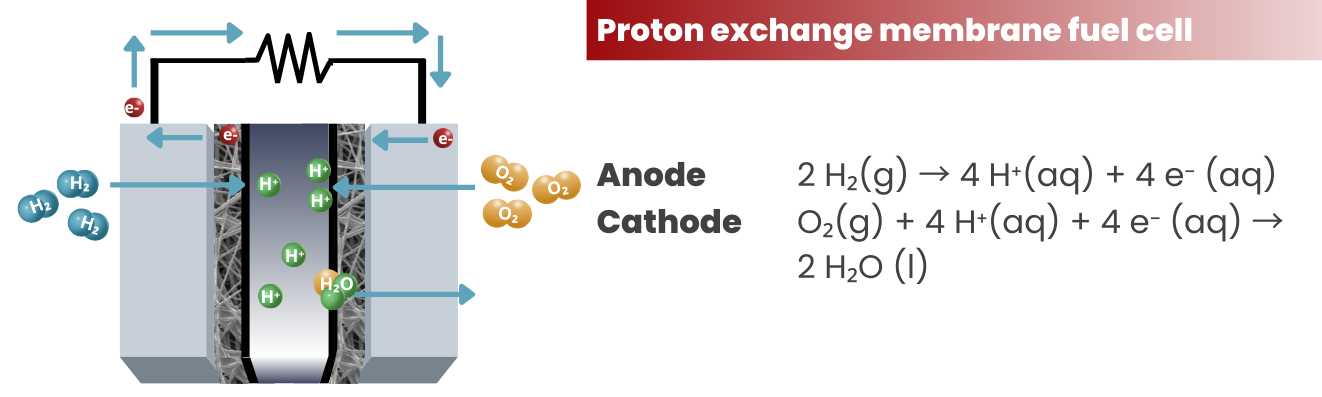
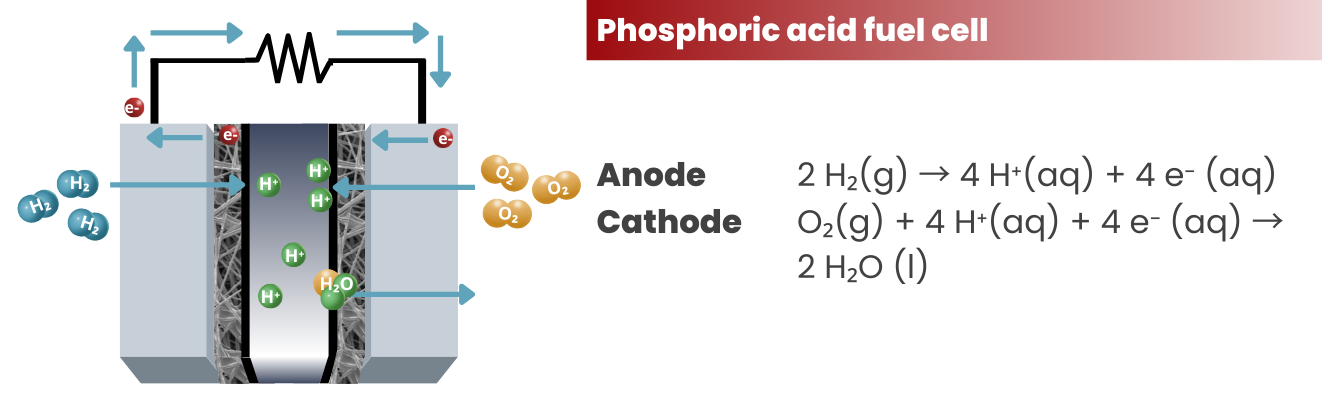
To expand a little bit on #3, electrons are stripped off from the atoms at the anode side. In the case of PEM fuel cells, H+ ions are produced according to the reaction:
2 H₂(g) → 4 H⁺(aq) + 4 e⁻ (aq)
The electrons and H+ ions will travel according to their designated pathways and will be used in the cathode for the reduction reaction:
O₂(g) + 4 H⁺(aq) + 4 e⁻ (aq) → 2 H₂O (l)
Hydrogen and Oxygen get in. Electricity (and water) gets out.
A fuel cell is a clean energy source with the only byproducts being electricity (power), heat and water. A single fuel cell alone only produces a few watts of power; therefore, several fuel cells can be stacked together to create a fuel cell stack. When combined in stacks, the fuel cells’ output can vary greatly, from just a few kilowatts of power to multi-megawatt installations.
You can generalize and say that it is a kind of battery that makes electricity on the fly. This is an example for the sake of simplicity. You can replace Hydrogen with any other suitable fuel and Oxygen with any other suitable oxidizing agent and you’d get the same result. Also what comes in/out depends on the type of Ion exchange membrane and catalyst that we are going to use.
Five Major Types of Fuel Cells (PEMFCs, SOFCs, DMFCs, AFCs, and PAFCs) and Their Applications
Fuel cells are electrochemical devices that convert chemical energy into electrical energy. There are several different types of fuel cells, each with its own unique characteristics and applications. Here are a few things about some of the most common types of fuel cells:
Proton Exchange Membrane Fuel Cells (PEMFCs): These are the most common type of fuel cells and are widely used in automotive applications. They use a polymer electrolyte membrane and require hydrogen and oxygen as fuel and oxidant, respectively. PEMFCs are known for their high efficiency, low emissions, and fast start-up times.
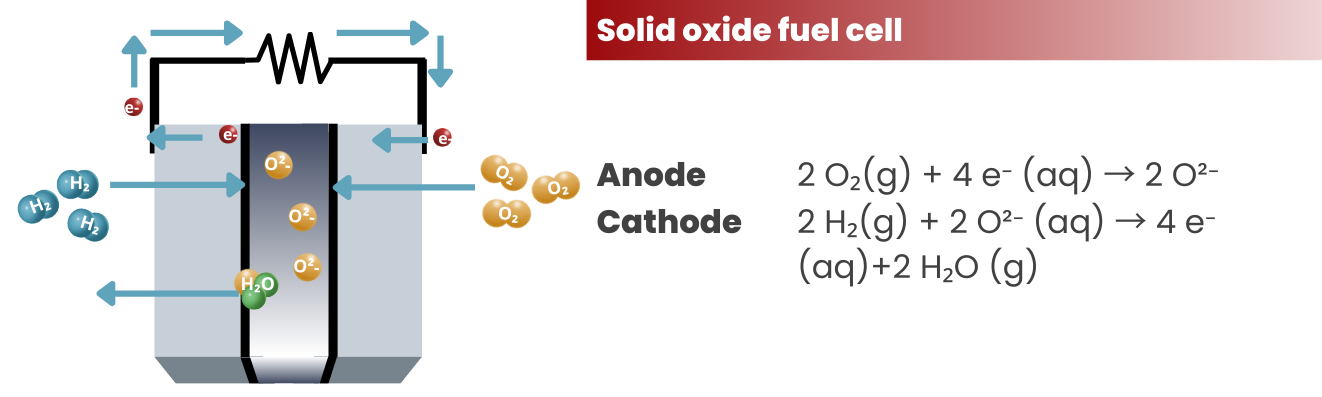
Solid Oxide Fuel Cells (SOFCs): These fuel cells operate at high temperatures (typically above 800 °C) and use a solid oxide electrolyte. They can operate on a variety of fuels, including hydrogen, natural gas, and biogas, making them suitable for a wide range of applications, including power generation and cogeneration.
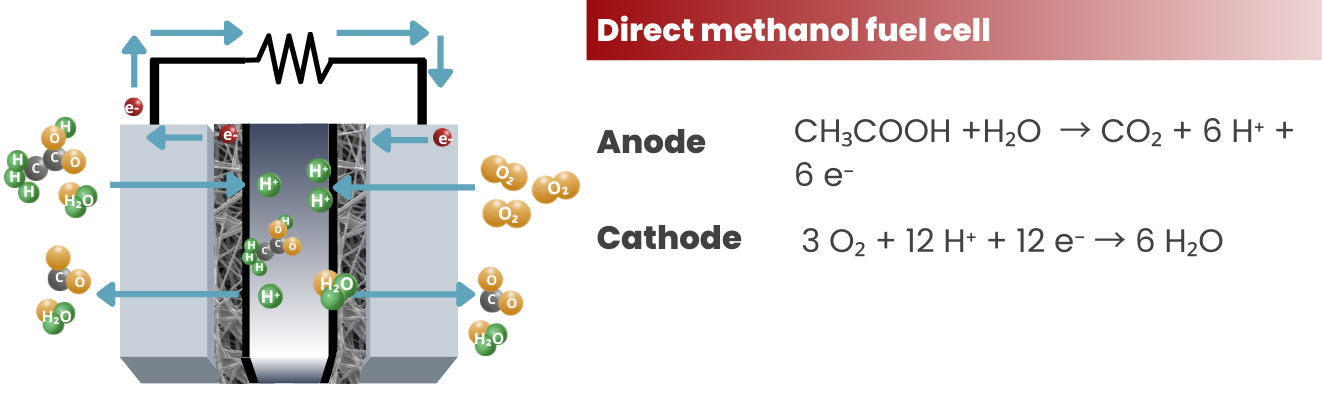
Direct Methanol Fuel Cells (DMFCs): DMFCs are similar to PEMFCs but use methanol as a fuel instead of hydrogen. They are typically smaller and more compact than PEMFCs and are used in portable applications such as mobile phones and laptops.
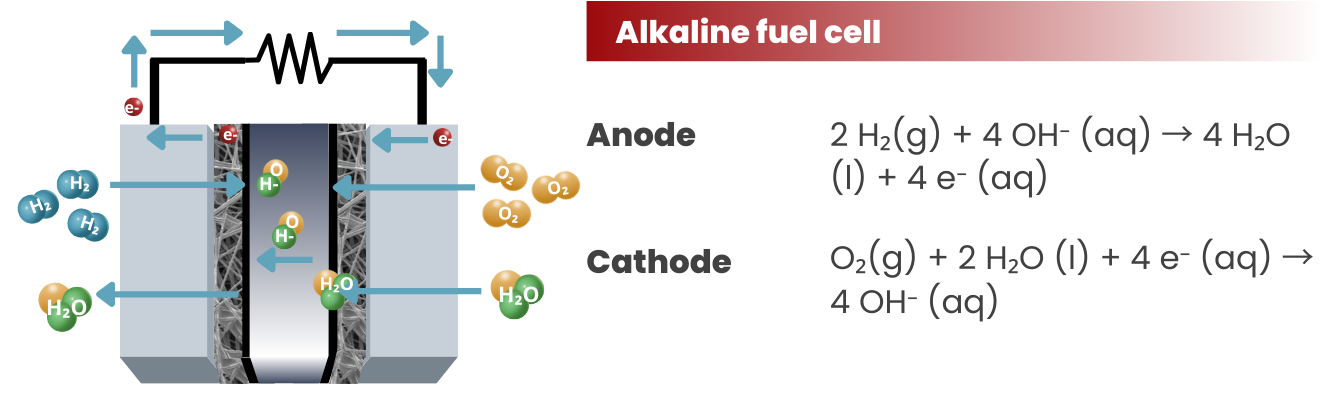
Alkaline Fuel Cells (AFCs): These fuel cells use an alkaline electrolyte (usually potassium hydroxide) and were among the first types of fuel cells to be developed. They are primarily used in niche applications such as spacecraft and submarines.
Phosphoric Acid Fuel Cells (PAFCs): These fuel cells use phosphoric acid as an electrolyte and operate at temperatures of around 150 °C. They are often used in stationary power generation applications, such as in hospitals and office buildings.
PAFCs share the same set of reactions with PEMFC. The only difference is the injection (and expulsion) of a phosphoric acid electrolyte in PAFCs
These are just a few examples of the many types of fuel cells that exist. Each type has its own unique characteristics and advantages, making them suitable for a range of different applications.
Thermal Management in Proton Exchange Membrane Fuel Cells
Heat generation within a PEMFC occurs in specific regions, resulting in a non-uniform temperature distribution. In the electrolyte, heat is produced due to the Joule effect caused by protonic resistance. At the membrane–electrode interfaces, localized heat arises from water sorption phenomena. Similarly, in the gas diffusion layers (GDLs), water condensation contributes to heat generation. On the electrodes, electrochemical reactions release heat, with approximately 50% of the total energy produced during the reaction between hydrogen and oxygen being converted into heat instead of electricity. This uneven thermal distribution impacts the overall performance and efficiency of the fuel cell.
Why is Thermal Management Important for Fuel Cells?
Thermal management is essential for keeping fuel cells durable and efficient. High temperatures can cause the membrane to dry out, leading to cracks or small holes, while low temperatures can result in flooding, reactant starvation, and damage to the catalyst layer. PEM fuel cells work best at around 80 °C, but researchers are developing high-temperature PEMFCs (over 120 °C) to improve reaction speed and make water management easier. However, operating at these higher temperatures can create problems, like reduced membrane stability, loss of hydration, and higher resistance. Materials like PTFE and ionomers may degrade near the membrane’s glass transition temperature. Other issues include the catalyst layer becoming rougher over time (Ostwald ripening), which reduces its active surface area, and hot spots forming in the cell, which can cause further damage.
Presentation at Thermal Management Expo 2024: Advanced Thermal Management Strategies for PEM Fuel Cells using Phase Change Materials
We are excited to share the recording and slides from our presentation at the Thermal Management Expo 2024, held in Germany from December 3-5. Titled "Advanced Thermal Management Strategies for PEM Fuel Cells Using Phase Change Materials," this presentation dives into innovative solutions for addressing the thermal challenges in PEMFCs.
In this session, we explore the role of phase change materials (PCMs) in maintaining optimal operating temperatures, enhancing fuel cell efficiency, and improving durability. The presentation covers key insights into how PCMs can mitigate temperature extremes, and reduce thermal gradients within the PEMFC stack. Beyond immediate applications, the presentation also explores avenues for future research, including optimizing PCM integration, scaling solutions for commercial systems, and developing advanced PCM formulations tailored to high-performance fuel cells.



 Why do fuel cells use thin GDLs?
Why do fuel cells use thin GDLs? Carbon papers for Fuel Cells & Electrolyzers
Carbon papers for Fuel Cells & Electrolyzers How Will the Impending PFAS-ban Affect Fuel Cells?
How Will the Impending PFAS-ban Affect Fuel Cells?