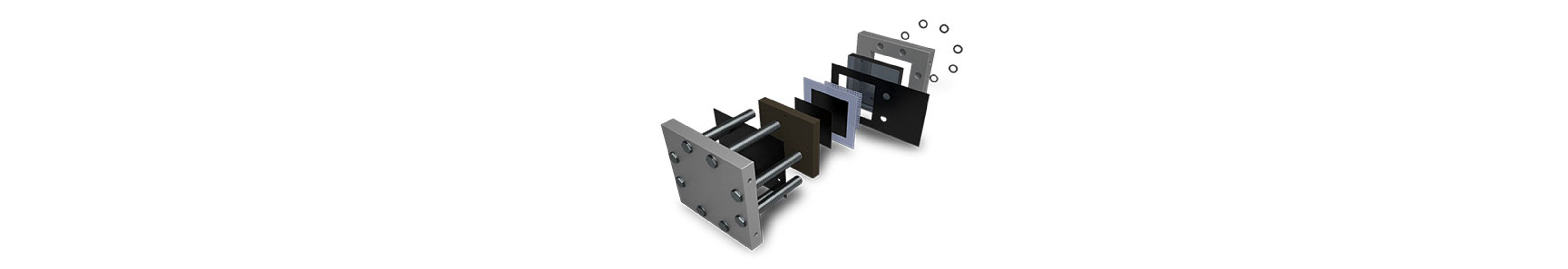
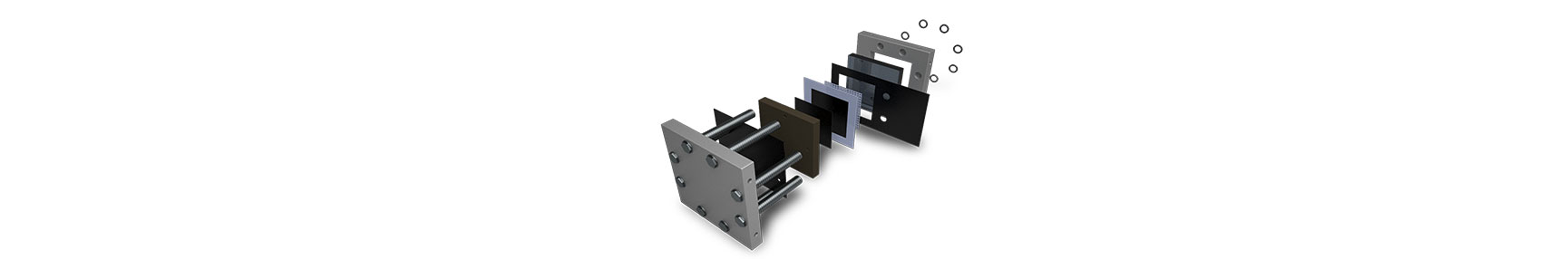
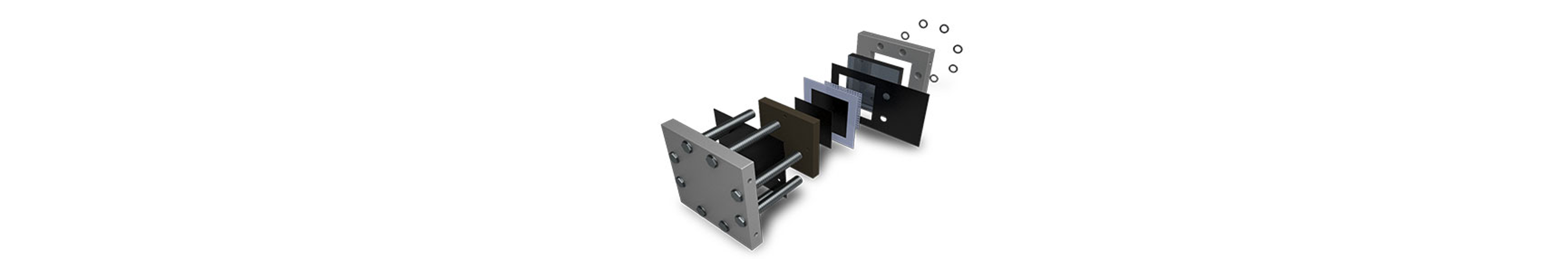
Water Electrolyzers
Frequently Asked Questions about Water Electrolyzers
Gas Diffusion Layers (GDLs) are crucial components in water electrolyzers, serving as porous substrates that facilitate efficient gas transport and distribution at the electrode interface. Carbon sheets are typically utilized on the cathode side for their electrochemical stability, high porosity, and hydrophobic nature. These properties ensure uniform gas distribution and prevent flooding of the cathode electrode during electrolysis.
On the anode side, metal-based Porous Transport Layers (PTLs) such as nickel, stainless steel, and titanium are commonly used. These materials offer corrosion resistance, mechanical strength, and high electrical conductivity, making them suitable for the oxidative environment of the anode compartment. Metal PTLs provide structural support to the anode electrode, enable efficient electron transfer, and ensure long-term durability under harsh operating conditions such as high temperatures and corrosive electrolytes.
How does porosity and pore size affect the performance of Porous Transport layer (PTLs) in water electrolyzers?
the porosity has a direct effect on the contact resistance between the MEA and the PTL. A small porosity increases the contact surface but hinders the oxygen expelling. On the other hand, for a given porosity, large pores facilitate gas/water transport but will increase the electrical resistance since contact points (forming the contact surface) are more distant from each other. Conversely, small pores will improve the electrical contact but gas expelling and water feeding will be obstructed, increasing the mass transport losses.
Untreated metal porous felts will not be consumed like a carbon Gas Diffusion Layer (GDL) will. However, the presence of oxygen does have an impact if the electrolyzer is being operated at high pressures (1 bar to 3 bars). The untreated metal surface will quickly form an electrically insulated oxide layer on the surface of the small-diameter fibers under high O2 pressures. This oxide coating will eventually affect the efficiency of the overall system acting as an electrical insulator and it will increase the interfacial resistance in the cell, lowering the electrochemical performance.
To prevent the formation of this detrimental oxide coating, applying a gold or platinum coating is recommended. This coating extends the lifetime of the metal felts, making it well-suited for applications requiring both high performance and long-term reliability. By preventing the formation of an oxide coating, stability in the electrochemical performance of the electrolyzer is greatly enhanced, ensuring consistent and efficient operation over its lifespan.
What is the difference between 1600°C and 2000°C graphitization?
Graphitization at temperatures around 2000°C typically results in enhanced electrical properties in carbon papers. However, this improvement often accompanies trade-offs, such as reduced compressibility or increased brittleness. Finding the optimal balance depends on understanding the specific requirements of your application.
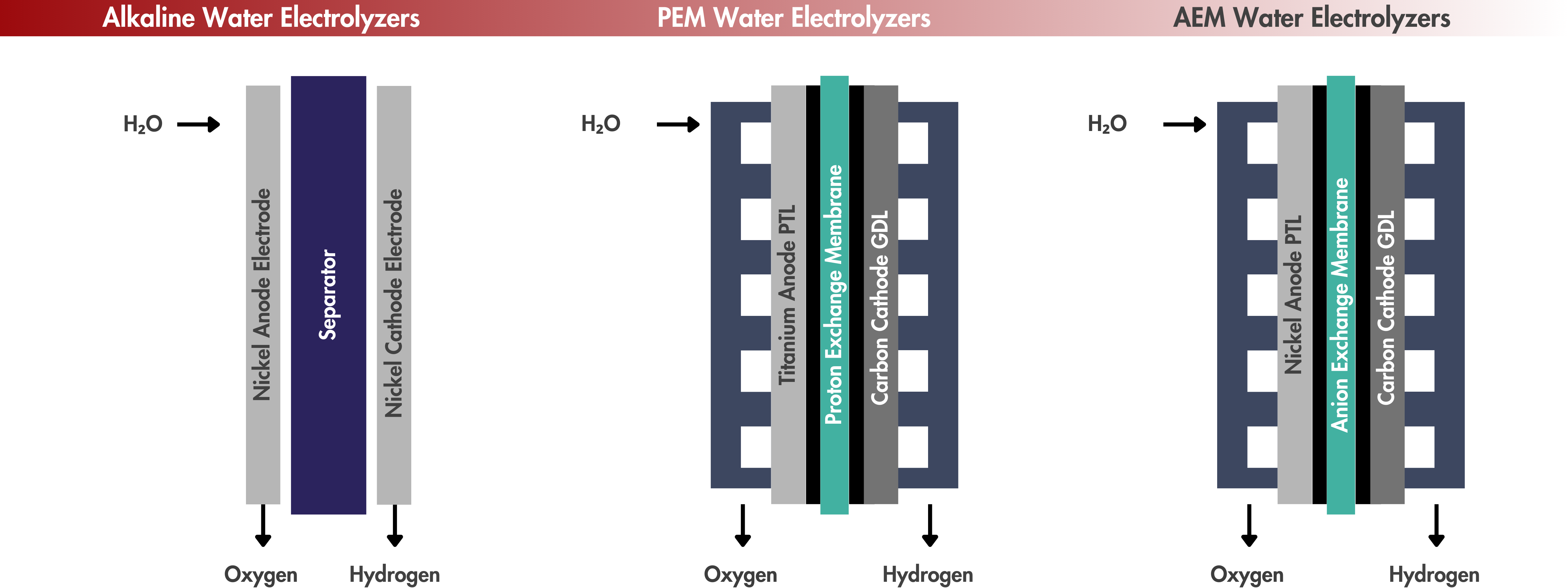
Types of Low-Temperature Electrolyzers
This presentation introduces the main types of low-temperature electrolyzers—systems that operate below 150 °C and are widely used for green hydrogen production. It covers the core technologies, including alkaline, PEM, and AEM electrolyzers, and highlights their operating principles, key materials, benefits, and challenges.
Related Blogs
Carbon papers for Fuel Cells & Electrolyzers
The blog article shown on the left is focused on the application of Carbon plates in electrolyzers ...
Product Catalogue
LINQCELLᵀᴹ Carbon Solutions for Redox Flow Batteries, Fuel Cells, and Water Electrolyzers
This catalog showcases LINQCELL Carbon solutions, designed for use as gas diffusion layers, porous transport layers, and electrodes in fuel cells, water electrolyzers, carbon dioxide electrolyzers, and redox flow batteries.
LINQCELLᵀᴹ Graphitized Carbon Fiber Plates for Water Electrolyzers
LINQCELLᵀᴹ Graphitized Carbon Fiber Plates are designed for use in water electrolyzers, offering excellent conductivity, durability, and corrosion resistance. Ideal for high-performance electrochemical applications, these plates enhance efficiency and reliability in hydrogen production processes.