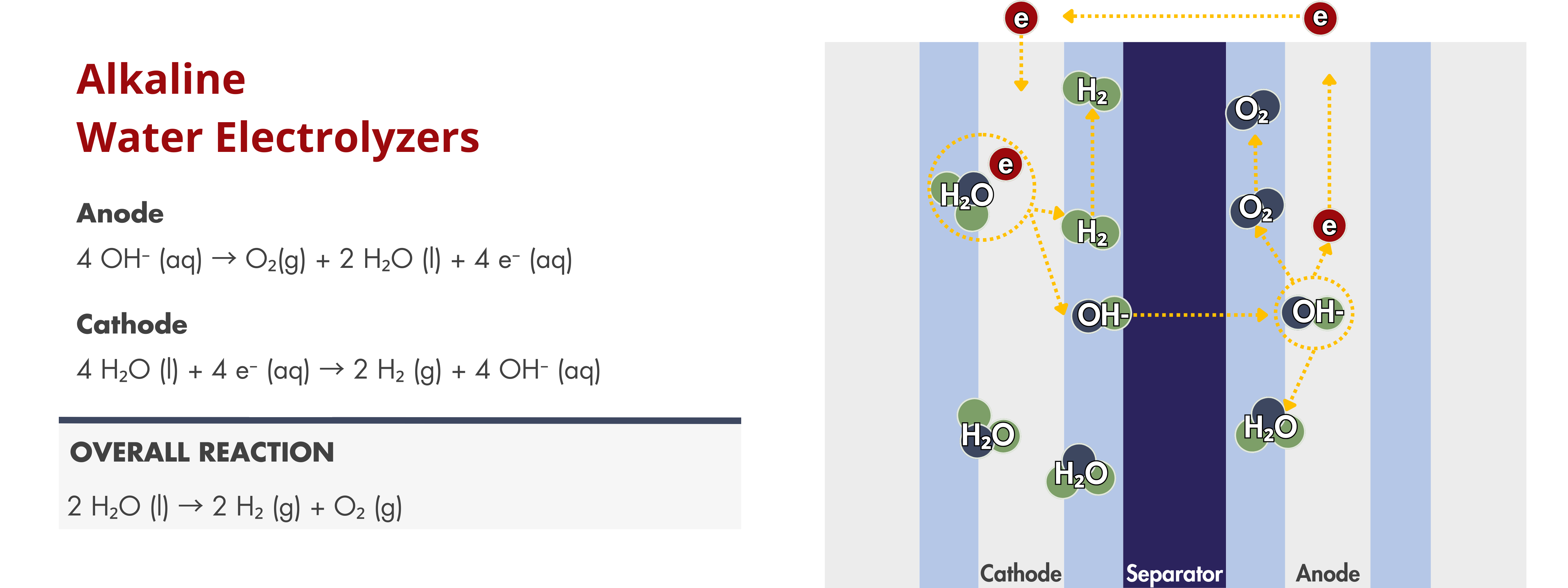
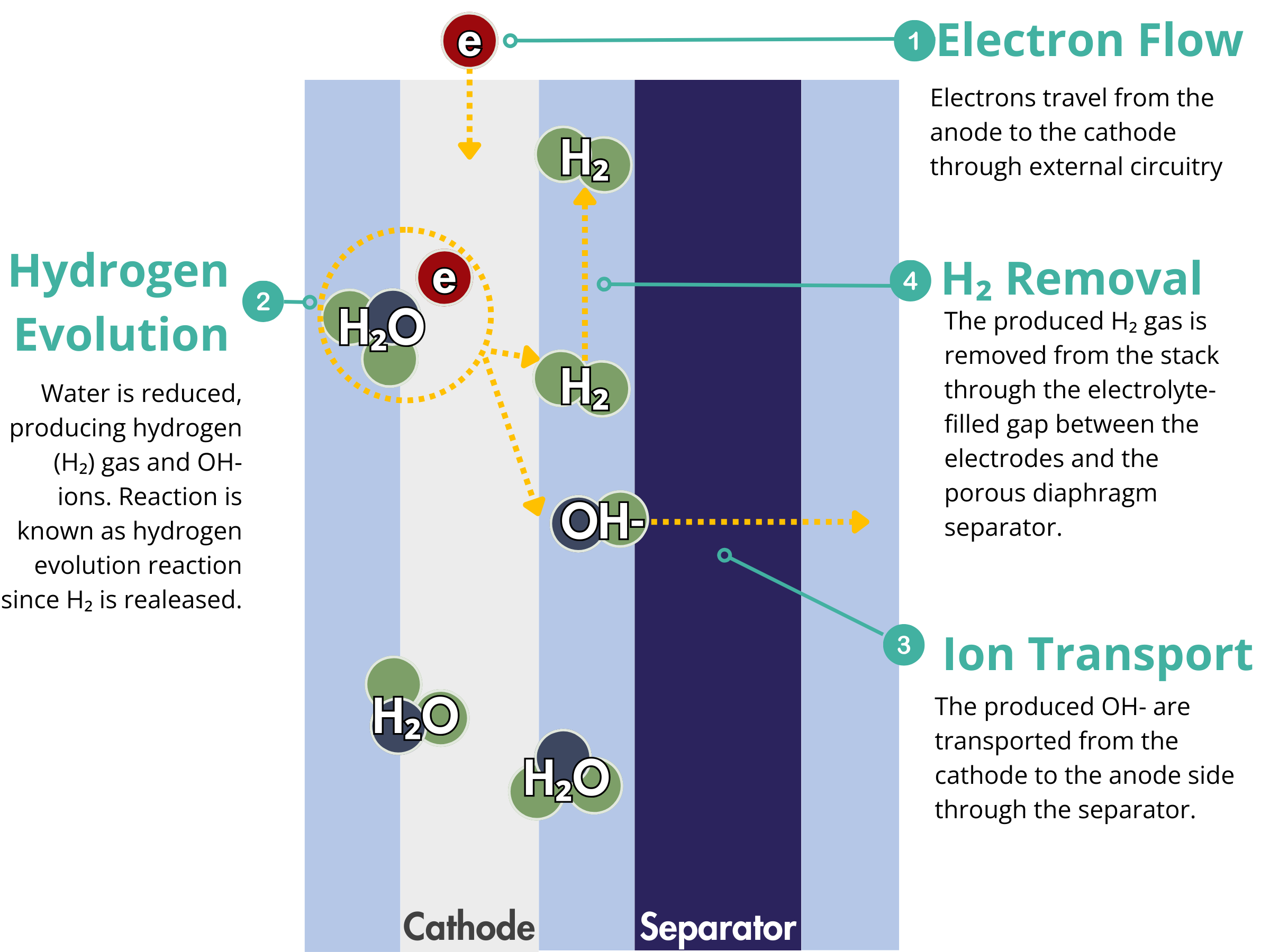
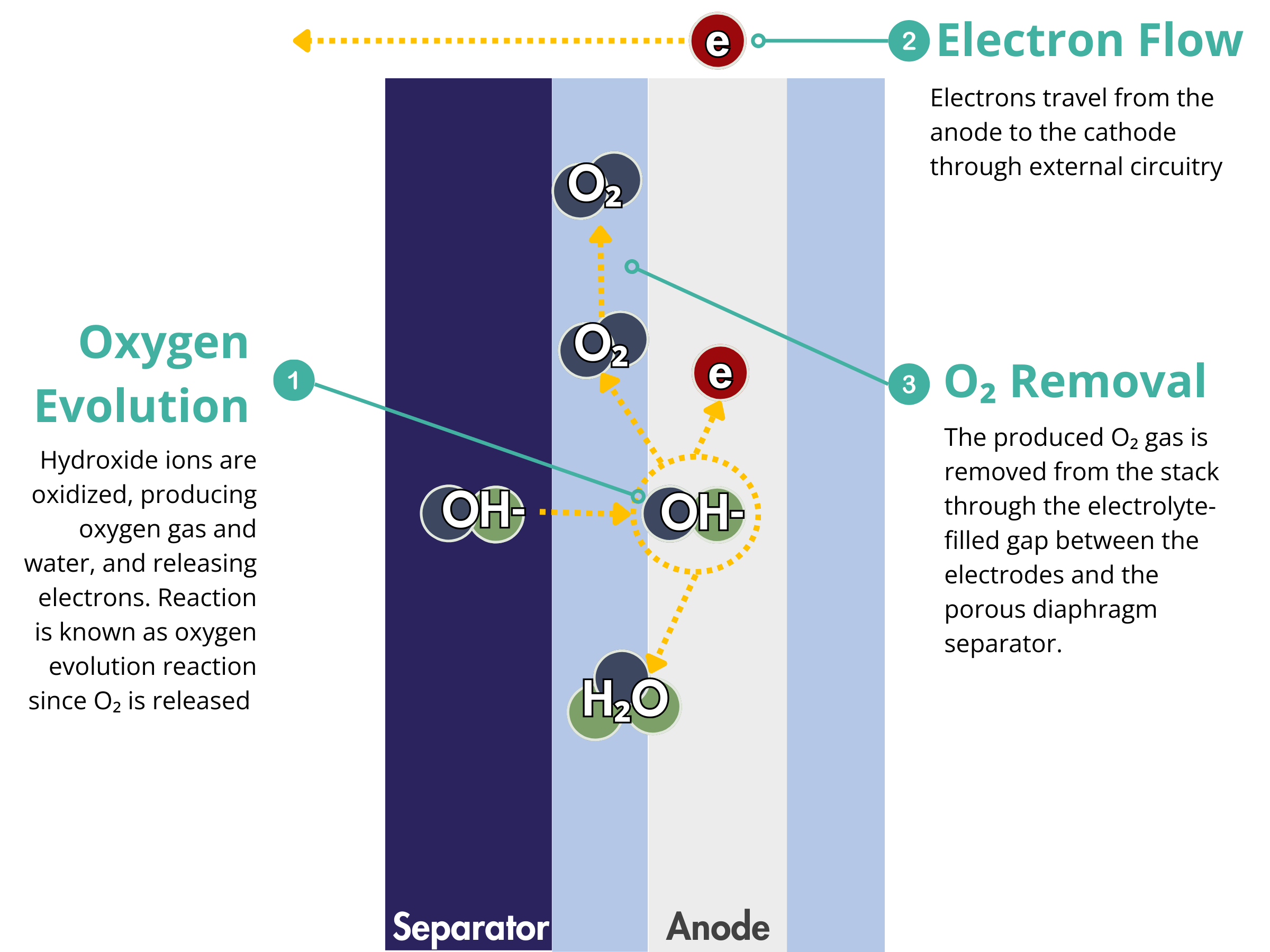
Alkaline Electrolyzers
LINQCELL ALK-PCS Porous Composite Separator for Alkaline Water Electrolyzers

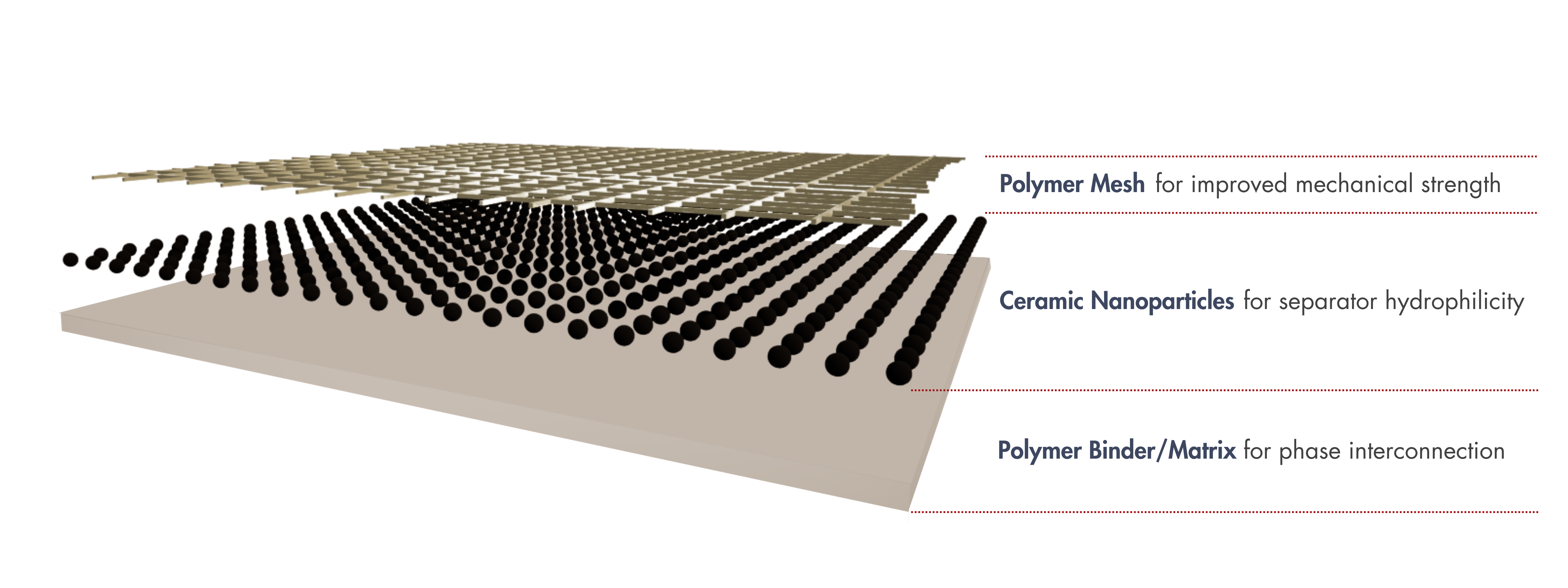
LINQCELL ALK-PCS is a composite separator made of a polyphenylene sulfide (PPS) mesh matrix reinforced with a ceramic nano coating. The PPS structure provides high mechanical strength and pressure resistance, making it ideal for high-pressure alkaline water electrolyzers. The ceramic nano coating enhances hydrophilicity, improving water management during electrolysis. Additionally, the polymeric binder with nanopores in the dense coating acts as a gas barrier, reducing gas crossover and enhancing operational safety.
| Property | Value | Unit |
|---|---|---|
| Thickness | 500±50 | µm |
| Porosity | 55±10 | % |
| Average Pore Diameter | <150 | nm |
| Maximum Pore Diameter | 200 | nm |
| Bubble Point | 3–5 | bar |
| Area Specific Resistance (80 °C, 30% KOH) | ≤0.15 | Ω·cm² |
| Maximum Operating Temperature | 110 | °C |
⚡ Applications
▪ Renewable energy
▪ Large-scale (1000 Nm3/h) and high-pressure hydrogen production
✅ Advantages
✔ Making electrolysis more efficient with superior gas barrier capabilities
✔ Making production highly scalable
✔ Withstands mechanical stress and pressure variations, ensuring long-term durability
📥 Downloads
⬇ Technical Data Sheet - LINQCELL ALK-PCS
Electrodes for Alkaline Water Electrolyzers
Electrodes are critical components in electrochemical devices, particularly in alkaline water electrolyzers, where they facilitate the electrochemical reactions at both the anode and cathode. At the cathode, they serve as the sites for the hydrogen evolution reaction (HER), while at the anode, they enable the oxygen evolution reaction (OER). The efficiency of these reactions directly impacts the overall performance of the alkaline water electrolyzer.
The first alkaline water electrolyzers used nickel-based electrodes, and these materials continue to be widely used today. Since AWEs operate in alkaline conditions, nickel and other transition metals are ideal electrode materials due to their excellent corrosion resistance and good electrocatalytic activity for hydrogen and oxygen reactions in alkaline environments. Nickel-based materials, such as Ni foams, Ni wire meshes, sintered Ni powders, and sintered Ni fibers, offer not only strong performance but also cost-effectiveness, as they are more affordable and abundant than precious metals. This makes AWEs an attractive option for large-scale hydrogen production, both in early applications and in modern systems.
Benefits of Using Nickel-based Electrodes in Alkaline Water Electrolyzers
HIGH CORROSION RESISTANCE IN ALKALINE CONDITIONS
Nickel has strong resistance to corrosion in the highly alkaline environment of water electrolyzers. This corrosion resistance results in reduced maintenance and longer-lasting electrodes.
HIGH ELECTROCATALYTIC ACTIVITY
FOR OER AND HER
Nickel has high catalytic activity towards HER and OER, reducing energy losses and increasing reaction rates.
ABUNDANCE AND LOW COST
Compared to precious metals, nickel is abundant and more affordable, making it a cost-effective choice for large-scale hydrogen production without compromising performance.
LINQCELL NICKEL Wire Mesh & Sintered Fibers for Alkaline Water Electrolyzers
Since electrodes are the key sites for electrochemical reactions in alkaline water electrolyzers, they are typically made from porous materials that provide a large surface area for efficient reaction kinetics. LINQCELL Nickel Wire Mesh and Sintered Fiber Felts are designed specifically to meet these needs, offering excellent corrosion resistance and high electrocatalytic activity.
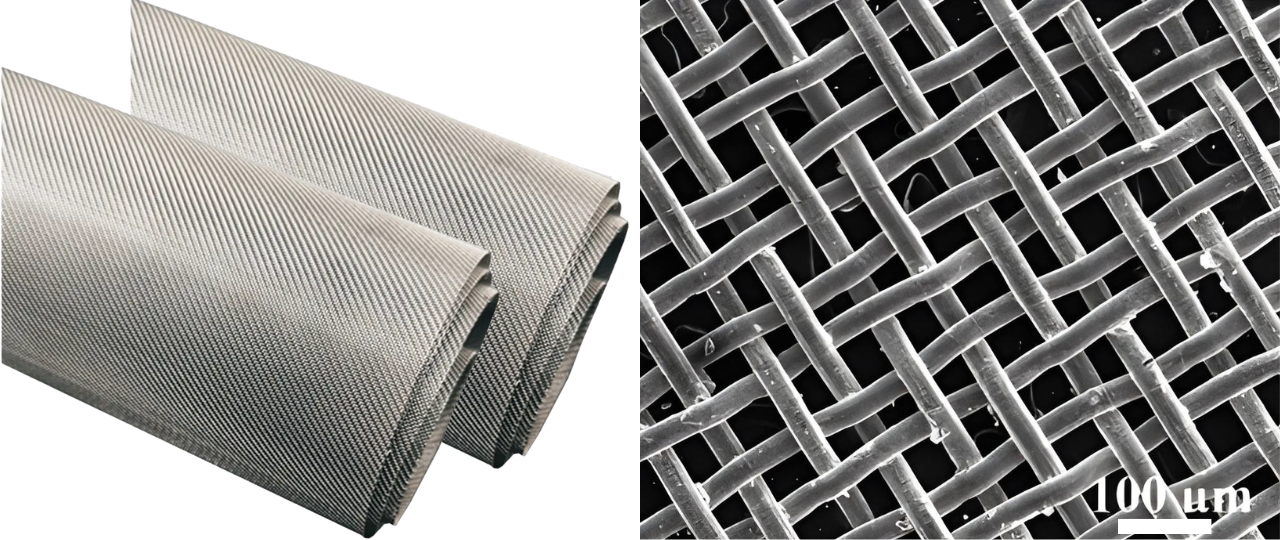
LINQCELL Nickel Wire Mesh is a high-purity, finely woven mesh available in two wire diameters: 0.25 mm (LINQCELL NWM250) and 0.30 mm (LINQCELL NWM300), offering superior electrical conductivity, corrosion resistance, and mechanical durability. With at least 99% nickel content and compliance with GB/T 17492, it ensures long-lasting reliability in demanding electrochemical applications. Available in mesh counts of 30, 40, 46, and 60, and two nickel grades (N4 and N6), LINQCELL Nickel Wire Mesh is ideal for precise tuning in alkaline water electrolyzers and other electrochemical devices.
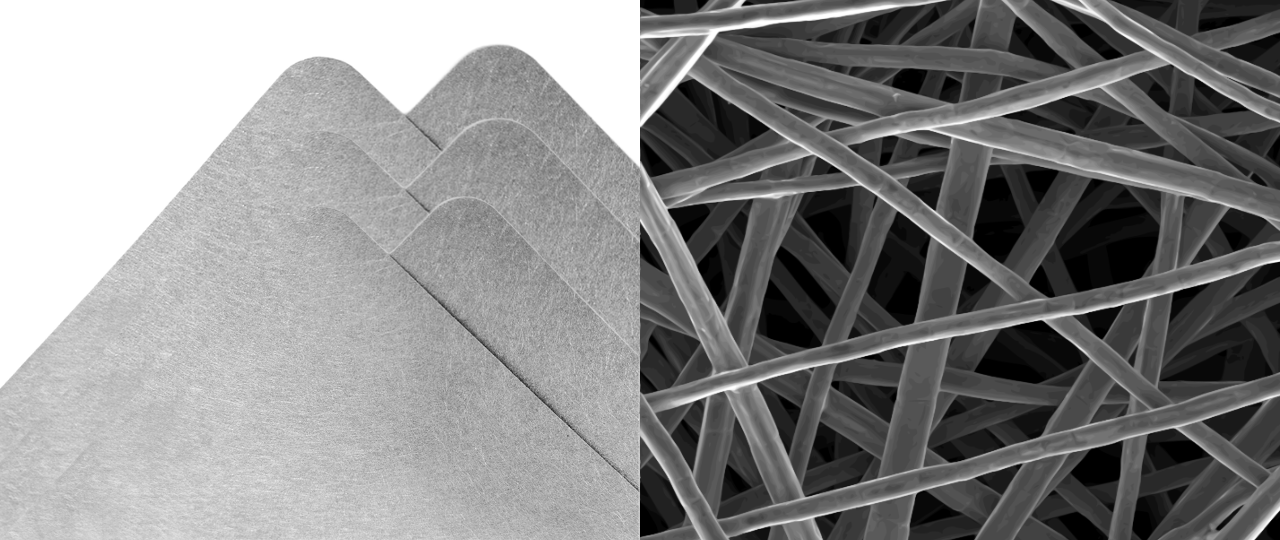
LINQCELL Sintered Nickel Fibers feature a unique three-dimensional porous structure, offering high porosity and specific surface area for efficient permeation and diffusion. These sintered fibers ensure consistent performance with their uniform pore size distribution. Made from high-purity Ni200 and Ni201, LINQCELL Sintered Nickel Fibers offer high corrosion resistance, water permeability, and efficient heat dissipation, making them ideal electrodes for alkaline water electrolyzers. They are available in two thicknesses: 250 µm (LINQCELL NFP250) and 500 µm (LINQCELL NFP500), and can be custom-made according to specifications.
Torn between LINQCELL Nickel Wire Mesh and Sintered Nickel Fiber?
Both materials are made from high-purity nickel, but their structures are different. Nickel wire mesh consists of woven metal wires, creating a flat, rigid grid with consistent openings, making it ideal for structural support and durability. In contrast, sintered nickel fiber is made up of small nickel fibers fused together into a spongy, three-dimensional network with higher surface area and porosity. The choice ultimately depends on your application.
⚡ Applications
▪ Alkaline water electrolysis
▪ Anion exchange membrane water electrolysis
▪ Nickel–metal hydride (NiMH) and nickel–cadmium (NiCd) batteries
✅ Advantages
✔ High electrical conductivity that minimizes overpotential
✔ High corrosion resistance that makes electrolysis cells durable
✔ Low cost that reduces capital expenditures
Frequently Asked Questions about Alkaline Water Electrolyzers
Alkaline electrolyzers use a liquid alkaline electrolyte and non-precious metal catalysts, whereas PEM electrolyzers use a solid polymer membrane and typically require precious metal catalysts like platinum or iridium. Alkaline systems are more established and cost-effective, while PEM electrolyzers offer higher efficiency and faster response times.
What are the advantages of alkaline water electrolysis for hydrogen production?Alkaline water electrolysis offers several advantages, including a lower capital cost due to its use of non-precious metal electrodes and mature technology. The system is built with durable materials that ensure a long operational lifespan, providing extended service life even under demanding conditions. Additionally, alkaline electrolyzers are highly scalable, making them suitable for both small-scale and industrial hydrogen production, meeting various production needs effectively.
While alkaline electrolyzers are traditionally designed for steady operation, newer designs with advanced electrodes and separators can handle intermittent power input, making them compatible with renewable energy sources like solar and wind.
What are the limitations of alkaline water electrolysis?Alkaline water electrolyzers have a few limitations, including slower response times, making them less suited for rapid power fluctuations. They also exhibit lower current density compared to PEM electrolyzers, which means that larger stacks are needed to achieve the same hydrogen output. Additionally, there are risks of gas crossover, necessitating the use of efficient separator materials to minimize losses and ensure safe operation.
Featured Presentation: CAPLINQ Product Offerings
CAPLINQ Materials for Alkaline Water Electrolyzers
Curious about how the right materials can improve electrolyzer performance? This quick presentation walks you through CAPLINQ’s lineup for alkaline water electrolyzers: what they’re made of, how they perform, and where they fit best. Whether you’re optimizing for efficiency, durability, or both, these materials are engineered to keep up.
Got questions or need help choosing the right materials for your alkaline water electrolyzer stacks? Reach out to us!
Contact Us →Presentations
Types of Low-Temperature Electrolyzers
This presentation introduces the main types of low-temperature electrolyzers—systems that operate below 150 °C and are widely used for green hydrogen production. It covers the core technologies, including alkaline, PEM, and AEM electrolyzers, and highlights their operating principles, key materials, benefits, and challenges.
Related Blogs

Benchmark Porous Transport Layers for Water Electrolyzers
This blog explores benchmark materials like carbon, titanium, and nickel used as porous transport layers in water electrolyzers, examining their material compatibility and stability under various operating conditions. It provides valuable insights into why nickel is the preferred electrode material for alkaline water electrolyzers.

What Are the Differences Between Sintered Metal Powders and Sintered Metal Fiber Felts?
This blog explores the differences between sintered metal powders and sintered metal fiber felts, focusing on their structure, permeability, and performance in various applications. It also provides insights into the structure of sintered nickel fibers, helping readers understand what makes them suitable for alkaline water electrolyzers.