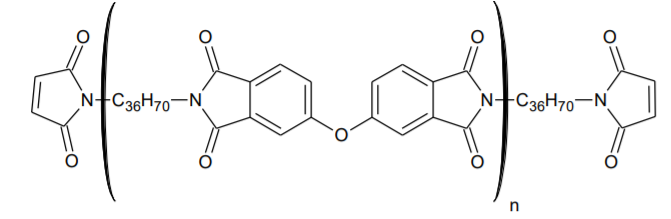
BMI-1500 Low Molecular Weight Bismaleimide Oligomer
- Soluble in many reactive diluents
- High adhesion to various substrates
- Superior thermal stability
Product Description
BMI-1500 is an amorphous, low molecular weight bismaleimide oligomer that exhibits good adhesion to a variety of substrates. It can be homo-cured via UV or free radical initiators to form tough, hydrophobic, cross-linked polyimides. The material has excellent low pH hydrolytic resistance and thermal stability. The amorphous nature of this imide-extended BMI allows it to form room-temperature-stable solutions in a variety of free radical reactive diluents. It is soluble in many common solvents such as toluene, xylene, MIBK, etc.
BMI-1500 is recommended for use as an additive or base resin in adhesives that are designed for high temperature resistance. It has excellent adhesion to a variety of substrates. When used as a base resin, it can produce adhesives that are tough, flexible and demonstrate good peel strength.
Technical Specifications
| General Properties | |
| Appearance Appearance Appearance at room temperature. | Amber, viscous liquid |
| Functionality | 2 |
| Molecular weight | 1504 g/mol |
| Physical Properties | |
| Viscosity Viscosity Viscosity is a measurement of a fluid’s resistance to flow. Viscosity is commonly measured in centiPoise (cP). One cP is defined as the viscosity of water and all other viscosities are derived from this base. MPa is another common unit with a 1:1 conversion to cP. A product like honey would have a much higher viscosity -around 10,000 cPs- compared to water. As a result, honey would flow much slower out of a tipped glass than water would. The viscosity of a material can be decreased with an increase in temperature in order to better suit an application | 20000 mPa.s |
| Thermal Properties | |
| Decomposition Temperature | >400 °C |
| Operating Temperature | 180 °C |
| Other Properties | |
| Storage Temperature | Room temperature °C |
Additional Information
BMI 1400 and BMI 1500 oligomers do not lend themselves to a similar "clean-up/purification" as we did with BMI-689. Despite that, 1400/1500 would likely benefit from the use of some coupling agents - especially as it relates to the post 85/85 adhesion values. These BMI resins are very hydrophobic and have high hydrolytic resistance, meaning water does not degrade the resin.
BMI resins, unless they are used with epoxies or other co-resins, do not have good adhesion to metals and they do have a large void volume. This void volume allows moisture to penetrate to the surface where the adhesion is not so good and further degrade it. However, with the proper use of coupling agents, the interface adhesion is improved and the 85/85 adhesion will follow.
What is the shelf life of the material?
DMI warrants the material for 1 year from the date of shipment when stored cold (+5ºC). This period is specified more to encourage customers to use the material and protect DMI from product returns as opposed to some chemical instability in the material. In fact, DMI stores the bulk BMI-1500 at room temperature prior to shipment. The absolute shelf life is many years - especially if stored cold and away from light - but we have never come up with a specific number of years.